I was delighted to be invited to attend a roundtable discussion event recently at the Cornwall council offices, hosted by the new chair of the board…
Case study: Stablepharma

The World Health Organisation estimates 50% of the world’s vaccines are rendered useless by the time they are administered. This is because they are thermally unstable and need to be kept between 2-8°C to work. Persistent failures in the global cold chain are the main cause of the wastage.
Dr Bruce Roser, inventor and founder of Stablepharma invented a patented solution that aims to change how the world combats disease with StablevaX Fridge Free vaccines.
Tell us about Stablepharma and how the initial concept came to be
The company is all about one man and his vision. The invention was originally discovered and developed by Dr Bruce Roser, who I like to call “our resident genius”. He was originally a clinical doctor by trade, but he’s got a very inquisitive mind. The eureka moment came when Bruce investigated the unique properties of a plant, the resurrection plant.
The plant senses a drought and shuts down by separating every single molecule in its body using a soluble and inert sugar. Having achieved a state of suspended animation, the plant is protected from drought. The key discovery by Bruce was that the plant was able to resurrect itself once the rains returned, something previously thought impossible.
Bruce then spent several years unpicking the science, working out how the plant works and found out that the plant was coating every individual cell in this soluble sugar called trehalose, which is organic sugar. He knew this could also work with drugs, and with a passion for saving lives, Bruce set to work on creating a way to thermally stabilise vaccines.
Bruce then used this knowledge to develop the world’s first ‘Fridge Free’ vaccine. Once you remove all the water out of a liquid vaccine, you are left with a powder. That’s now stable. Add water, it comes back to life in a few seconds, and it’s good to inject.
This can make a huge difference to the success rate of vaccination programmes and that’s where our mission comes in. The main benefit of the invention is that vaccines can be stockpiled without the need for refrigeration. Moving medicine and drugs around is a huge logistical operation which can be virtually impossible in certain parts of the world. By irradicating the need for the cold chain those logistical issues all but disappear and there are massive environmental benefits too.
What challenges have Stablepharma faced?
The biggest problem we have had over the years is that ‘others’ just don’t believe what we have done is actually possible, it’s the ‘too good to be true’ syndrome, or in some cases ‘we didn’t think of it, so it can’t be any good’ attitude! However, having recently produced validated data showing we have kept a StablevaX Td vaccine at 45°C for one year has been a game changer in terms of acceptance and credibility.
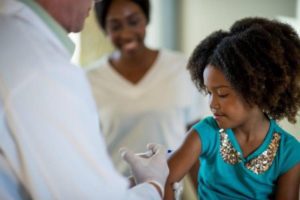
As we all know, Covid-19 posed problems for the entire world with devastating consequences, but for our industry the upside was that it exposed the exact problem we’re trying to fix, and all of a sudden, the whole world was aware of the logistical issues facing vaccination programmes and vaccines in general. With awareness of the problem, the better it becomes understood globally and the more support we have in our mission to fix it. I think it was John Harvey Jones (ICI Chairman) who once said ‘’knowing that you have a problem, is 75% of fixing it’’.

Stablepharma has had such an interesting journey so far. What do you consider to be one of the biggest successes or milestone moments?
There have been a few champagne moments on our journey so far, but one in particular that sticks out for me is when we asked the MHRA (the UK Medical Health Regulation Authority) to run informal trials for us on our first vaccine conversion to the StablevaX format and received a ‘pass mark’. We knew then that this was no longer a moon-shot project!
We’ve also had several meetings with the World Health Organisation over the years, who have been very supportive with guidance on the correct protocols that help you map out the final product with confidence, the end game being that the product will have value.
What is next for Stablepharma?
Now we’ve proven our product will comply to regulatory standards and it works, we’ve had engagements with various well known vaccine manufacturers who are interested in licencing our technology. Our goal isn’t to develop new vaccines, but to collaborate with manufacturers to convert their current approved vaccines to a ‘Fridge Free’ status.
Working with
PKF Francis Clark
The team at PKF Francis Clark (Bristol) are great at getting things done. We overshot our normal eligibility, but the team were able to upgrade our status for EIS (enterprise investment scheme) and helped us obtain additional grant funding. It was very impressive and we’ve really enjoyed working with the team to date.
Because of the growth potential we want to work with an accountant we know can handle that growth, as well as help us prepare for any potential acquisitions. We know Paul Bray and the team at PKF Francis Clark can support us going forward and we are very pleased with the service we receive.
You can find out more about Stablepharma here.
© All photos provided by Stablepharma
Further information:
Talk to one of our advisers now and see what we could do for your business.



